Immunofluorescence
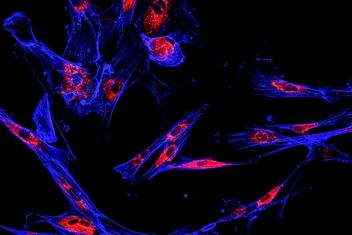
Immunofluorescence (IF) is an immunostaining technique that combines the use of antibodies with fluorescence imaging to visualize specific proteins and other biomolecules within a tissue or cell sample. IF is a powerful technique that can be used with a variety of samples including fixed whole cells, paraffin-embedded cell pellets and frozen or paraffin-embedded tissue samples.
Immunofluorescence Resources
What is Immunofluorescence?
IF is an immunoassay technique that uses antibodies labeled either directly or indirectly with a fluorophore to visualize proteins and other biomolecules. Though the terms IF and immunohistochemistry (IHC) are often used interchangeably, they have grown to have distinct meanings, with IHC denoting the use of an enzymatic reaction resulting in a visible precipitate to visualize an antibody. Since its initial development, IF has grown into a powerful tool enabling the visualization and colocalization of many proteins and biomolecules within a single tissue with an accompanying array of fluorophores that cover the entire visual spectrum.
How Does Immunofluorescence Work?
IF protocols can be divided into three major steps: sample processing, staining, and imaging. Sample processing occurs prior to staining and typically involves fixing tissue samples in a cross-linking agent, such as paraformaldehyde, to preserve tissue morphology. Tissues are then dehydrated and embedded into paraffin blocks enabling tissues to be cut into tissue sections and affixed to a glass slide for long-term storage. When slides are ready to be stained they are processed in a clearing agent to remove excess paraffin and are rehydrated.
Staining protocols usually start with a blocking step consisting of either a protein or serum block to reduce non-specific binding. Sections are next incubated with an antibody specific to the protein or biomolecule of interest. At this point, there are two main types of IF protocols: direct and indirect. If the primary antibody is conjugated with a fluorophore then it is considered direct IF. An example of indirect IF is when the primary antibody is unconjugated and a targeted secondary antibody is conjugated with a fluorophore. Indirect IF has the advantage of increasing signal enabling the detection of less abundant proteins, but at the cost of increased complexity and potential background.
Once slides are finished staining, they are then imaged with either an epi-fluorescent, confocal, or multispectral microscope. Depending on the number and type of fluorophores used it may be necessary to do spectral unmixing to ensure that each marker is accurately quantitated. Although there is a plethora of fluorophores to choose from, many have overlapping emission spectra limiting their use in multiplexing. Spectral unmixing is a powerful technique that distinguishes fluorophores not only from other fluorophores being used that have overlapping spectra but even background fluorescence.
2-Step Protocols
Use the following protocols to assist your immunofluorescence testing:
- Epitope Retrieval Method for FFPE tissues and cell blocks
- Formaldehyde-Fixed Cells and Cytospin Preparations
- Formalin-fixed, Paraffin-embedded tissues and cell blocks
- Cells Grown in Culture and Cytospin Preparations
- Fresh Frozen Tissue Sections
Related Applications
Why Use Immunofluorescence?
IF is a powerful technique when there is a need to visualize multiple proteins within a single sample. With advances in confocal microscopy and multispectral imaging it is now possible to stain more than 9 antigens in a single tissue, which is impossible with traditional IHC.
IF also allows users to understand co-expression and spatial relationship between multiple proteins and biomolecules through co-localization while still preserving tissue context that also is not possible with IHC.
Immunofluorescence FAQs
Immunofluorescence staining refers to the technique that is used to detect and visualize a range of molecules within a sample. The process of staining consists of four main steps - cultivation, fixation, staining and imaging.
Immunofluorescence is used to determine whether cells or tissues within a sample are expressing the antigen under investigation. For samples when antigens are present, it is possible to use immunofluorescence to determine which subcellular compartments are responsible for the antigen expression.
Immunofluorescence can be split into two different classifications - primary (or direct) and secondary (indirect). Primary IF is classified by the use of a single, primary antibody to recognize the target antigen, whereas secondary IF requires two antibodies - with direct and indirect binding to the antigen.
By clicking “Acknowledge”, you consent to our website's use of cookies to give you the most relevant experience by remembering your preferences and to analyze our website traffic.
